BROOMFIELD, CO – Dr. Zung Vu Tran began his long career of aggregating, organizing, and standardizing clinical studies for the benefit of medical practitioners in 1985, when he authored the first meta-analysis ever published in the Journal of the American Medical Association (JAMA). Then, after three decades of working as a biostatistician, Dr. Tran approached Michael Willis, a tech entrepreneur in Boulder, Colorado, with an idea. Why not use existing technology to give clinicians immediate access to the latest medical research?
“The problem is that physicians aren’t current,” Willis explained during a recent interview with CyberMed News. “That’s not to blame them. There’s a lot of research being done and physicians are busy people. They have little time to read much of it.”
After several months spent discussing the potential technology, Willis and Dr. Tran, alongside their colleagues William Kent and Neil Cox, founded MedAware Systems, a Broomfield, Colorado-based digital health company. The prospect of an app that could instantly perform a meta-analysis of medical research excited many investors, and the company was quickly funded.
“There are approximately 1.5 million research studies conducted every year worldwide. A little more than a hundred thousand of these are clinical trials that examine treatment efficacy. Until now there hasn’t been a way to give physicians and patients easy access to that information, much less to interpret it.”
Having completed two years of development and beta testing, MedAware Systems recently began piloting its meta-analysis technology, which promises to reduce the time it takes medical research to enter clinical practice from a decade to a minute.
Standardizing the Data
By systematically reviewing the research on a particular condition or treatment, a meta-analysis provides practitioners with a comprehensive sense of current best practices in the clinical setting. But meta-analyses are often published years after the studies they review have been performed.
According to Willis, one of the reasons for this delay is the variety of measures researchers use to quantify data and present findings. While recently creating a standardization algorithm for neurological studies, Dr. Tran encountered over three thousand different measures, each with its own scale.
“The problem with these measures is they’re scaled differently. Some go from thirty to seventy, and as the numbers increase, the outcome is better. Others go from five to minus five, and as the numbers decrease, the outcome is better.”
After developing a standardization algorithm for a particular field of medicine, MedAware Systems will empanel three to four experts in that field. These experts will then give MedAware Systems an understanding of what practitioners would most want to know from the hundreds of published studies they review with the company.
“Some things will be very general, like the name of the journal, the authors, its date of publication, and information about the people who participated in the clinical trial. Then it gets very specific, focusing on treatment outcomes and how they’re reported.”
Once a framework has been established, the medical research analysts of MedAware Systems will read through thousands of studies in that particular field of medicine, filling out the framework for each. To ensure the data they transfer remains accurate, the analysts follow a rigorous process of double-blind entry.
“We’re aggregating, organizing, and standardizing all of the information from human clinical trials in one place. That’s a big task. At this point, we’ve read more medical research than anyone on the planet.”
Supporting Clinical Decisions
Clinicians currently have several ways to access the latest medical research. They can read journals like JAMA, the Lancet, and the New England Journal of Medicine. They can perform a search on PubMed or even the Internet. But all of these methods can be time-consuming and, worst of all, imprecise.
“If you want to get information quickly, these are not the best tools,” Willis explained. “The reality is that whether you’re a patient or a physician, you’re spending a lot of time and effort trying to get information on treatment efficacy.”
MedAware Systems addresses this problem by giving clinicians immediate access to current research in a quick-to-read and easy-to-understand format. Using the company’s app, clinicians can search by condition or treatment to find the information they need to make the best decisions for their patients.
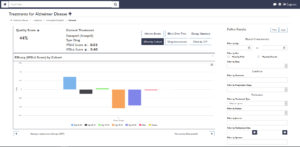
“If I’m interested in helping one of my dementia patients with their memory, I can bring up the number one selling drug for Alzheimer’s and look at its efficacy in that area. If I discover that the research on its efficacy is inconclusive, I can access the study that delivered these results and learn why that’s the case.”
At the push of a button, Dr. Tran’s algorithms allow clinicians to perform meta-analyses on the medical research MedAware Systems has aggregated, organized, and standardized. The ability to immediately know where current research stands on treatment efficacy has been viewed by many as a game-changer.
“With MedAware Systems, you don’t have to wait five years for the experts to put a meta-analysis together. You can perform one instantly.”
Like the coverage that CyberMed News provides? Follow us on Twitter, LinkedIn, and Facebook to make sure you keep up to date on the most recent developments in digital health.






Be the first to comment on "MedAware Systems Is Bridging The Gap Between Medical Research And Clinical Practice"